
MPX-RESPONSE project
Enhances Europe’s capacity to provide a rapid response to the mpox international outbreak
Last updated on 04 February 2026
In brief
MPX-RESPONSE aims to increase the knowledge of mpox, to evaluate potential treatments, and to strengthen Europe’s global preparedness for emerging infectious disease outbreaks.
Origin of the MPX-RESPONSE project
In 2022, the multi-country outbreak of mpox (Monkeypox) virus disease was declared a public health emergency of international concern by the World Health Organization (WHO). One year later, in July 2023, the end of the emergency was announced but ongoing reported cases have been kept under surveillance as the threat of new outbreaks remains real. More recently, since November 2023, an unprecedented mpox epidemic has been reported the Democratic Republic of Congo (DRC) and extended to several African countries, caused by various viral clades and in particular by newly identified clade Ib, which led the WHO to declare again a PHEIC linked to mpox on 14 August 2024.
A better understanding of the disease will allow effective strategies for patient management and treatment, preventing a global health crisis.
MPX-RESPONSE project partners
This project involves 16 leading international scientifc partners:
- ANRS MIE /INSERM,
- Oslo Universitetssykehus HF, Oslo, Norway
- Universita degli studi di Verona, Verona, Italy
-
Inserm Transfert SA, France
- Assistance Publique des Hôpitaux de Paris, Paris, France
- Servicio Madrileno de Salud, Madrid, Spain
- European Clinical Research Alliance on Infectious Diseases, Utrecht, Netherlands
- Universitair Medisch Centrum Utrecht, Utrecht, Netherlands
- Universiteit Antwerpen, Antwerpen, Belgium
- Erasmus Universitair Medisch Centrum Rotterdam, Rotterdam, Netherlands
- Fundacao Oswaldo Cruz, Brazil
- Norwegian Institute of Public Health, Folkehelseinstituttet, Norway
- PANTHER, France
- Universitat Basel, Basel, Switzerland
- University of Geneva, Geneva, Switzerland
- Fundación Huésped, Argentina
Methodology and studies of the MPX-RESPONSE project
Based on WHO « Core » protocol
In response to the 2022 outbreak of mpox disease, the World Health Organization (WHO) has promoted a structured and coordinated approach to prevent redundant research efforts and expedite the assessment of treatments against mpox disease. A group of experts, convened by the WHO and ANRS MIE, has been tasked with developing a core protocol, designed to promote global collaboration. Trials seeking to support this collaborative exchange are encouraged to adopt standardized inclusion criteria and endpoints. The UNITY, MOSA and EPOXI protocols were developed based on the WHO core protocol. While inclusion criteria are adapted to the local context, all three studies share the same primary endpoint, enabling possibility to pool data and promptly reach more robust conclusions.
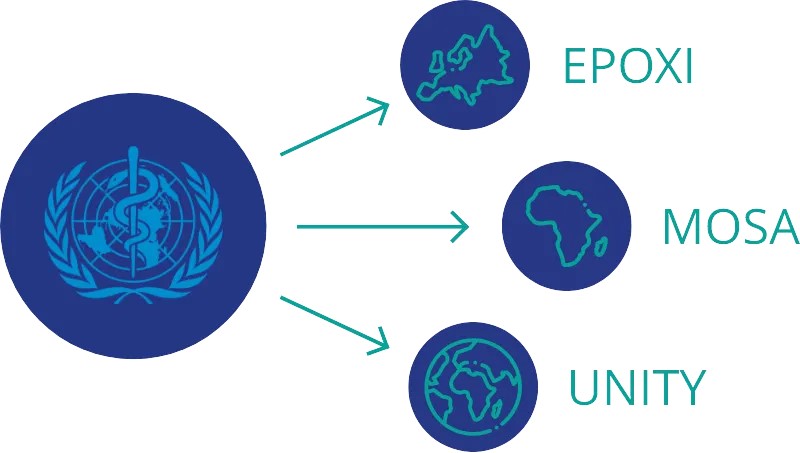
The MPX-RESPONSE project includes three randomized clinical trials (RCTs)
The protocols of all three trials are based on the WHO CORE protocol, designed for a global trial assessing drug safety and efficacy in treating human mpox (tecovirimat), with the goal of securing marketing authorization from the EMA, if the treatment demonstrates positive results.
- UNITY trial : Coordinated and sponsored by ANRS MIE. Geographical coverage: International (Argentina, Brazil, Switzerland)
- MOSA trial : Coordinated and sponsored by PANTHER. Geographical coverage: Sub-Saharan African countries (endemic mpox regions)
- EPOXI trial : Coordinated by Ecraid and with Universitair medisch centrum Utrecht as scientific sponsor. Geographical coverage: Europe
The MPX-RESPONSE project include one cohort : MOSAIC cohort
The objective of this observational study is to deepen our understanding of the clinical and virological outcomes in patients diagnosed with mpox. This approach aims to provide a comprehensive insight into the natural history of the disease. This cohort, in addition to European Union countries, is conducted in United Kingdom and Switzerland. Oxford University is the sponsor of the study and ANRS MIE the sponsor representative in European Union.
Through MPX-RESPONSE, our goal is to increase the knowledge on mpox and to evaluate treatments to better fight against the disease in Europe and globally, thanks to the unique international dimension of the consortium. A methodology designed to promote collaboration harmonises inclusion criteria and primary endpoint across the three clinical trials in MPX-RESPONSE, enabling data pooling and ultimately allowing to reach more robust conclusions in a timely manner.
Organisation
MPX-RESPONSE is organized into 4 Work Packages (WP)
- WP1 : COORDINATION, lead by ANRS MIE
- WP 2 : MOSAIC COHORT, lead by Assistance Publique des Hôpitaux de Paris
- WP 3 : EPOXI TRIAL, lead by European Clinical Research Alliance for Infectious Diseases (Ecraid),
- WP 4 : UNITY AND CLINICAL STUDIES, lead by Oslo University Hospital
MOSA: brincidofovir, an antiviral candidate against mpox, passes initial test
As part of the MOSA trial, coordinated and sponsored by PANTHER, the first antiviral tested, brincidofovir developed by Emergent BioSolutions, poses no health risk after an initial randomisation of 50 patients since 2024 in the Democratic Republic of Congo. The MOSA Data Safety and Monitoring Board therefore recommends continuing the trial.
This adaptive, double–blind, placebo–controlled trial plans to recruit 50 more patients (adults and children, particularly those living with HIV) during the first half of 2026, in order to reach the next milestone for the interim analysis of brincidofovir’s efficacy.
To know more
