
PROACT EU-Response
Preparing Europe to respond to future pandemics by creating a network of adaptive clinical trials
Last updated on 19 December 2025
Main points
- PROACT EU-Response aims to strengthen Europe’s capacity to respond effectively to future health emergencies by establishing a network of adaptive clinical trials.
- The consortium is led by ANRS Emerging Infectious Diseases and Inserm.
- The project will run for five years, from January 2025 to January 2030, with a budget of €17 million. It has received funding from the European Union’s Horizon Europe research and innovation program (grant agreement No. 101156304).
PROACT EU-Response: responding better to the threat of emergencies
Recent health crises, and in particular the COVID-19 pandemic, have highlighted the urgent need for faster and more coordinated responses to pandemics. PROACT EU-Response aims to strengthen Europe’s capacity to respond effectively to future health emergencies by establishing a network of adaptive clinical trials.
Discover PROACT EU-Response’s consortiumA brief history
Conceived in 2023, PROACT EU-Response follows on from the EU-Response project, coordinated by Inserm, which brought together 22 partners and 16 countries from 2020 to 2026.
EU-RESPONSE‘s objectives included:
- Extending the DisCoVeRy study, a phase III, open-label, adaptive, randomized, controlled, multicenter clinical trial designed to evaluate the safety and efficacy of drugs in hospitalized adult patients diagnosed with COVID-19, to many European countries. The DisCoVeRy trial was initially launched in France as a complementary study to the WHO’s Solidarity trial.
- The establishment of a new European multinational adaptive clinical trial platform: the EU-SolidAct platform.
PROACT EU-Response seeks to consolidate and increase the scope of what was established during the EU-Response project.
Key figures
PROACT EU-Response: different fields of work
In order to prepare Europe to respond to future pandemics, PROACT EU-Response has multiple objectives that are interrelated and complementary (see the full list of working groups below).
Establishing networks
One of PROACT EU-Response’s objectives is to expand a network of clinical trial sites across Europe to test therapies for respiratory viral infections and ensure rapid responses during pandemics. This network of clinical centers includes 80 centers in 21 European countries.
In addition, a network of nine microbiology laboratories, called VIRvOLT, enables the entire network of clinical centers to develop and share diagnostic and monitoring tools. These tools will be used to perform specialized biological analyses and identify biomarkers in order to measure the effectiveness of treatments and improve understanding of antiviral resistance.

The main objective of PROACT EU-Response is to prepare Europe to respond extremely quickly and in a coordinated manner to a health crisis. The project aims to establish a platform for therapeutic trials capable of testing the efficacy of new molecules within a very short timeframe.
In crisis situations, this platform will be able to evaluate treatments against emerging pathogens in real time, providing rapid and informed responses to health authorities and healthcare professionals, thereby strengthening Europe’s resilience to future pandemics.
Social sciences at the heart of the project
PROACT EU-Response also has a component dedicated to social sciences, seeking both to understand the drivers of misinformation in times of crisis and to improve health literacy among European patients and citizens. The goal is to build a network of social science researchers capable of providing a nuanced understanding of social contexts, and to establish a community group responsible for carrying out activities aimed at raising awareness among European patients and citizens about scientific and health issues.
Some of the topics covered in this project include:
- The importance of including civil society in recruitment assistance for therapeutic trials in pandemic situations;
- Understanding the barriers to patient participation in therapeutic trials;
- Assessing literacy on how therapeutic trials work and on the evaluation of new molecules in pandemic situations;
- The beliefs of citizens in Europe about emerging infectious diseases.
These actions will ultimately contribute to the development of training modules for healthcare professionals, better inform public policy in times of crisis or between crises, and lead to the creation of an algorithm for detecting early signs of radicalization or misinformation about a new health phenomenon.
An adaptive platform for therapeutic trials
One of the main objectives is to set up a platform for therapeutic trials called EU-SyndAct (for “Syndromic Adaptive Controlled Trials”), which will be able to respond quickly during a health crisis to determine the effectiveness of molecules against the pathogen responsible for the crisis (see Section 2 below).
Discover EU-SyndActANRS Emerging Infectious Diseases’s role
ANRS Emerging Infectious Diseases is responsible for the overall coordination of this consortium. In addition to coordinating the project, ANRS MIE will also be the sponsor of the SyndAct-1 clinical trial (see Workstream 2 below).
PROACT EU-Response working groups
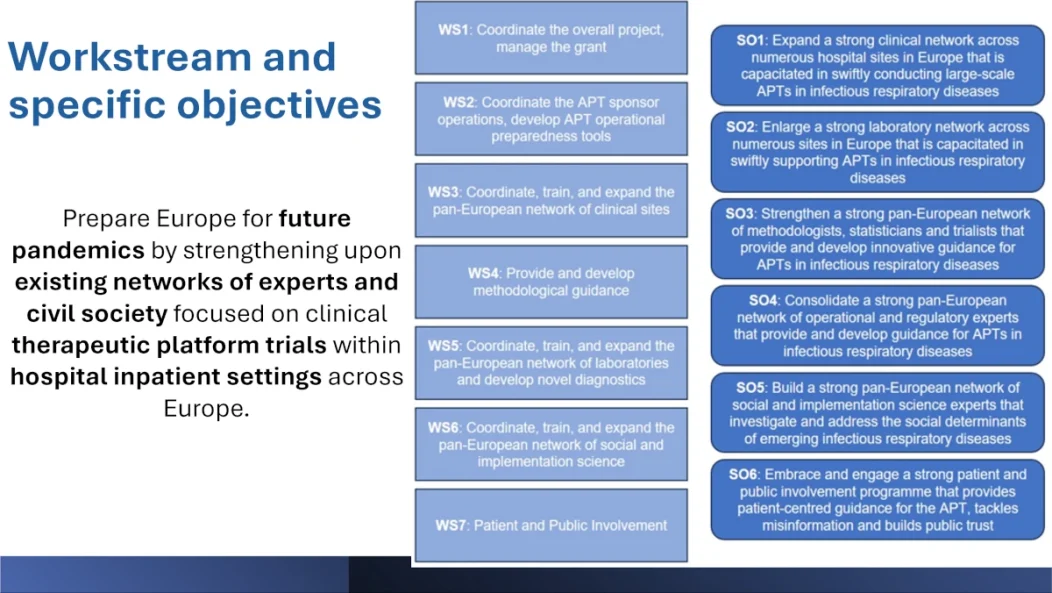
Workstream 1: Project coordination and impact
Workstream 1 is responsible for the overall coordination of the project and managing collaboration between the 25 partners in 11 countries. It is responsible for administrative, financial, and technical monitoring in order to meet European Union requirements.
Workstream 2: Management of the adaptive testing platform and preparation tools
Workstream 2 focuses on the management of the adaptive clinical trial platform and the development of tools for preparing large-scale multi-country trials. It will also address safety monitoring, reporting, and the transition from EU-SyndAct-1 to EU-SyndAct-2.
Workstream 3: Clinical trials and investigations network
Workstream 3 focuses on expanding a network of clinical trial sites across Europe to test therapies for respiratory viral infections and ensure rapid responses during pandemics. Key activities include identifying and expanding trial sites, building capacity through site assessments and training, and creating master protocols for trials targeting existing and emerging pathogens.
Workstream 4: Methodological capacity and statistical analyses
This working group is dedicated to providing robust statistical and methodological support for platform trials within the PROACT EU-Response framework. It is divided into several sub-working groups:
- Statistical design, data management, and analysis for EU-SyndAct trials to ensure optimal protocols and execution.
- Development of methods for real-time data sharing between trials and rapid evidence generation, enabling accelerated decision-making.
- Integration of observational studies and cohorts through emulation of target trials, to improve knowledge of antiviral treatments.
- Development of core outcome sets to standardize efficacy measures in trials, facilitating data comparability as well as systematic reviews and meta-analyses.
Workstream 5: Personalized biological analyses: European network of laboratories and identification of biomarkers
Workstream 5 focuses on specialized biological analyses, biomarker identification, and personalized biological analyses for immunological research to measure treatment efficacy. It relies on the expansion of the European network of virology laboratories VIRvOLT to perform standardized viral load tests and advanced biological analyses during and after clinical trials. Biomarker research focuses on virological, immunological, and genetic markers to guide personalized treatment strategies and improve understanding of antiviral resistance.
Workstream 6: Social sciences and implementation sciences
Workstream 6 focuses on integrating social sciences and implementation to optimize trial design, recruitment, and engagement, particularly for marginalized and underrepresented groups. Key activities include synthesizing qualitative data, analyzing media discourse, and conducting rapid qualitative research to gather real-time information during trials.
Workstream 7: Capacity Building, Community Involvement, and Communication
Workstream 7 focuses on capacity building, improving communication, and promoting community involvement to ensure trial readiness and effective dissemination of trial results. Key objectives include preparing trial sites through tailored training, combating misinformation, and building trust among communities. The component emphasizes the creation of community advisory committees and patient empowerment to actively involve them in trial design and dissemination of results. Dissemination efforts will target multiple audiences, including patients, policymakers, and healthcare professionals, to ensure broad awareness and meaningful impact of trial results.
Discover also

Strive: An international network of clinical trials
Strive is an international network of clinical trials on infectious respiratory threats and viral emergences funded by the National Institutes of Health (NIH).
17 June 2025
