
EU-SyndAct: a platform for adaptive clinical trials
EU-SyndAct, which stands for “European Syndromic Adaptive Clinical Trial,” is an adaptive clinical trial platform that aims to strengthen Europe's capacity to respond effectively to future health emergencies.
Last updated on 19 December 2025
Main points
- EU-SyndAct (which stands for “European Syndromic Adaptive Clinical Trial”) is a European platform for adaptive clinical trials, designed to respond to the challenges posed by respiratory viral infections, whether endemic or emerging.
- These trials feature a new methodological approach (umbrella protocol) that accelerates the evaluation of new therapies while maintaining high scientific and statistical rigour.
Background
The declaration of COVID-19 (30 January 2020) and then mpox virus disease (23 July 2022) as public health emergencies of international concern by the WHO served as a reminder that emerging infectious diseases remain a major threat to public health. In the face of climate change and international trade, new health threats are likely.
These crises have also led to the emergence of new forms of clinical research, conducted “in real time” and “in the real world“, to accelerate the evaluation of treatments for emerging infectious diseases.
Hence the need for proactive approaches that ensure the rapid availability of medical countermeasures. PROACT EU-Response therefore aims to strengthen Europe’s capacity to respond effectively to future health emergencies by establishing a network of experts and civil society focused on setting up adaptive clinical trials. One part of the project will be the management of the EU-SyndAct adaptive clinical trial platform and the development of tools for preparing large-scale multi-country trials.
The objectives of the EU-SyndAct trials are:
- To understand diseases caused by potential new infectious agents
- To establish a rapid and coordinated response to pandemic threats,
- To Effectively evaluate new treatments to make them available to patients quickly, particularly in the event of the emergence of new pathogens.
EU-SyndAct trial: a specific methodology
Platform trial based on an umbrella protocol
The methodology of an adaptive umbrella-type platform trial provides a flexible, effective and harmonised framework for simultaneously evaluating several experimental treatments for hospitalisation-level respiratory tract viral infections within a single global protocol. The study design allows several interventions to be evaluated simultaneously in a clinical trial.
The platform study design is based on:
- An umbrella protocol covering several sub-studies specific to each pathogen (e.g. EU-SyndAct 1 will focus on influenza, Covid-19 and RSV). The presence of a single common control group for several sub-studies reduces the number of participants to be evaluated in order to make decisions on the effectiveness of the interventions. In addition, the harmonisation of the methodology and analyses allows for comparison between the different arms.
- Parallel randomisation: Patients are randomised between several treatment arms (experimental treatment vs standard care) according to the pathogen identified.
- New methods of data analysis: Continuous assessment of efficacy (futility assessment) after including a sufficient number of participants will enable treatment to be stopped early if there is a low probability of achieving its objectives (futility) or, conversely, for treatments deemed not futile, to move on to phase 3 with a larger number of participants in order to confirm their efficacy.
Thanks to its flexible and adaptive nature, this type of protocol makes it easy to add or remove treatment arms as knowledge advances and the scientific and epidemic context evolves.
The target population will be hospitalised adults (≥18 years of age) with severe respiratory viral infections (coronavirus, RSV, influenza, or other emerging pathogens).
The primary objective will be to evaluate the efficacy of treatments (new or repurposed molecules, alone or in combination) on the recovery of hospitalised patients, compared to standard care. Secondary objectives will also include the evaluation of safety and the risk of resistance.
Discover PROACT EU-Response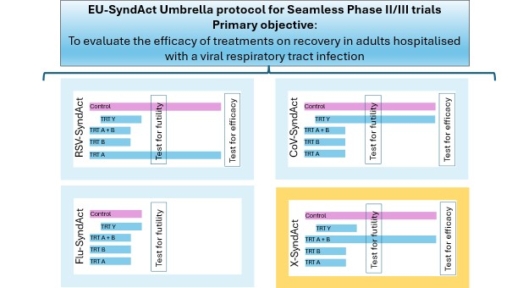
Next steps
The project is currently in the development phase. The next steps will consist of finalising, in collaboration with experts in the field, the drafting of the protocols for the various sub-studies, as well as the selection of the molecules to be evaluated.
At the same time, coordination with the partners involved in the project and the selection of participating clinical centres will continue.
Once these elements have been defined, the necessary regulatory procedures can be initiated, and the project can be rolled out operationally with a view to including the first participants.

