Oropouche
Oropouche virus disease is an arboviral disease actively circulating in several regions of Central and South America and the Caribbean.
Last updated on 05 May 2025
In brief
Oropouche virus disease is is an arboviral disease actively circulating in several regions of Central and South America and the Caribbean. Since 2023, the Oropouche virus has caused major outbreaks in endemic regions, but also in previously unaffected areas.
Origin of the Oropouche virus
History: a look back at the Oropouche outbreak
The Oropouche virus (OROV) was first detected in 1955 in Trinidad and Tobago in the Caribbean, near the Oropouche river.1 Since then, it has mainly affected South America and Latin America (Brazil, Panama, Peru, Argentina, Bolivia, Colombia, Ecuador and French Guiana).2-5
From 2023 onwards, the virus caused major outbreaks in historically endemic regions, but also in previously unaffected areas.6 Although it did not result in any serious cases, the outbreak which began in Cuba in June 2024 marked an unprecedented geographical expansion of the virus. It reached the United States and Europe (Germany, Italy, Spain, the Netherlands and France) in August 2024.7
Direct, horizontal transmission of the virus has never before been documented. In addition, as of 30 July 2024, five cases of possible vertical transmission (i.e. from mother to child during pregnancy) have been identified in Brazil, resulting in four stillbirths.6 The potential risk during pregnancy and effects of the infection on the foetus are currently being studied.8
Current state of knowledge
Oropouche virus disease is an arboviral disease caused by OROV, a single-stranded RNA virus belonging to the Orthobunyavirus genus in the Peribunyaviridae family.
Transmission
OROV is transmitted to humans mainly by the bite of small midges of the genus Culicoides paraensis, which live in humid forest areas. After infection, the incubation period varies from 3 to 10 days.
Symptoms
Symptoms are often non-specific and are quite similar to those of other arboviroses such as Dengue, Chikungunya or Zika. Patients may present with fever, headaches, nausea, joint and muscle pain, conjunctivitis and abdominal pain. However, around 80% of those infected remain asymptomatic. Symptoms typically last for up to one week, but in some cases it can extend over several weeks. In around 4% of symptomatic cases, serious, neuroinvasive complications may occur, including meningitis and encephalitis
Diagnosis
Diagnosis is based on several methods. Detection of the virus by RT-PCR is possible between D1 and D7 after the onset of symptoms. Serological tests such as ELISA can also be used to detect IgM and IgG antibodies, produced from day 1 to 2 weeks after the onset of the disease. The biological samples used for these tests include serum, saliva and urine. In patients showing signs of neuroinvasive disease, cerebrospinal fluid may also be analysed.
Treatment and prevention
To date, treatment is essentially supportive, involving rehydration and administration of analgesics and antipyretics. In order to reduce the risk of haemorrhage, as with dengue fever, aspirin and other non-steroidal anti-inflammatory drugs are not recommended. Several antiviral candidates have been tested, but none has been shown to be effective against the virus. Although it has been effective against other Orthobunyaviruses. ribavirin has shown no antiviral activity against OROV in vitro or in vivo.
There is no vaccine to prevent Oropouche infection.
Research avenues
Transmission
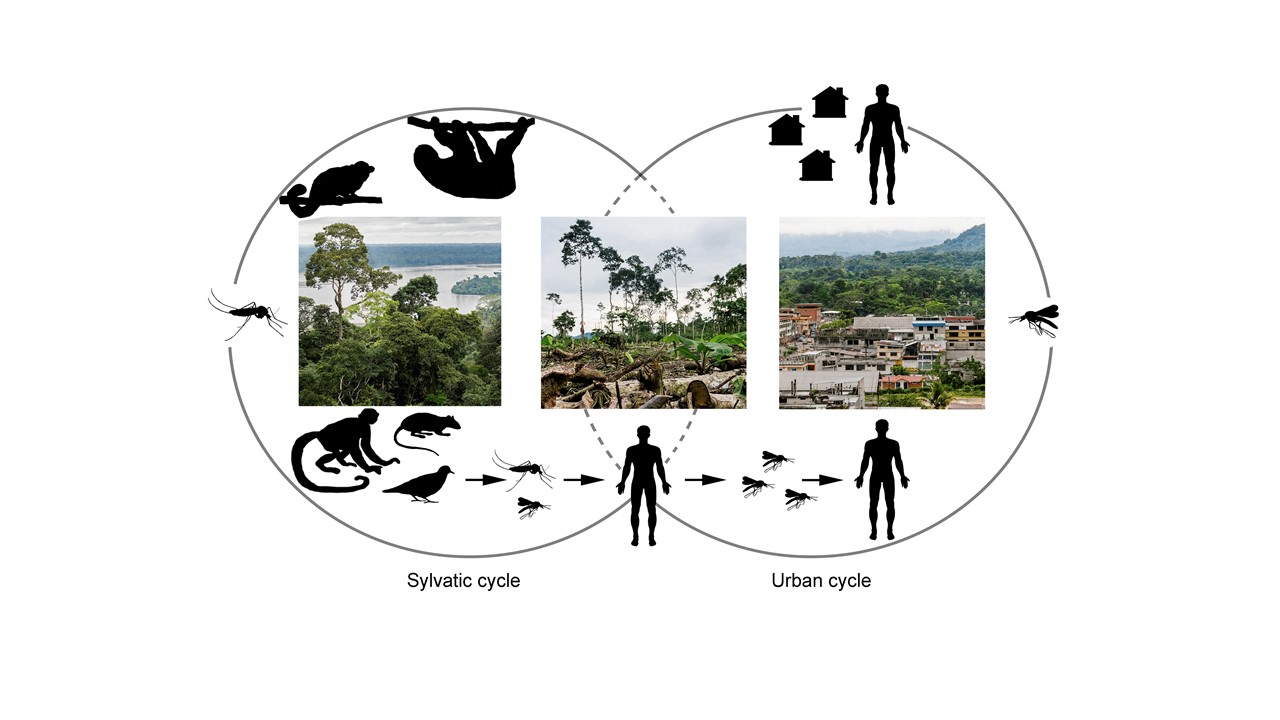
Sylvatic and urban cycles of the Oropouche virus are still poorly understood.
Sloths, non-human primates and wild birds are suspected to act as natural reservoirs for OROV. However, their precise role is not known. It is also essential to investigate other as yet unknown natural hosts, accidental hosts and potential amplifiers, including domestic animals.
Vector competence in the life cycle of OROV has been poorly explored. While Culicoides parensis continues to be the main species studied, other species found in populated areas, such as Culex quinquefasciatus, Aedes serratus and Coquillettidia venezuelensis, should be the subject of further research.
This suggests that studies into vector competence and seroprevalence in humans, and the strengthening of entomological surveillance in infected regions, would help to better characterise the actual OROV circulation. Surveillance could benefit from research into the presence of arboviruses in wastewater to identify the level of circulation in human populations and anticipate epidemics of OROV and other arboviruses.9
As mentioned above, the routes of OROV transmission are also poorly understood: as with the Zika virus, vertical transmission from mother to foetus seems possible.8
Pathogenesis
Knowledge of the pathogenesis of OROV and the risk factors associated with severe forms is also limited. Although the majority of infections are asymptomatic, certain populations such as the elderly, pregnant women, immunocompromised populations or those with co-morbidities appear to be more vulnerable. In addition, congenital OROV infections and their possible association with neonatal anomalies such as microcephaly are still poorly documented, despite recent cases.8
The clinical signs therefore should be investigated, as do the long-term sequelae in individuals infected with OROV, and the correlation between prolonged viremia or a high viral load and the severity of the infection.10
Diagnosis
At present, Oropouche is a largely under-diagnosed disease. Clinical symptoms are confused with those of other arboviroses such as Dengue, Chikungunya and Zika, and require laboratory confirmation, thus delaying diagnosis. Developing rapid diagnostic tests for OROV is needed to enable early identification of the virus.
Determining the most appropriate sample for diagnosis remains a major challenge. In-depth studies of the viral load in various bodily fluids (saliva, blood, urine) and the limits associated with their analysis are therefore essential.
Diagnosis of OROV infection by neonatal ultrasound has begun to be tested with the Institut Pasteur. In 2024, the Pan American Health Organization (PAHO) published recommendations on laboratory detection and case surveillance for OROV.11
Treatments and vaccines
To date, no specific antiviral treatment is available, and very few clinical trials have been conducted in humans. Favipiravir has not yet been tested for OROV, but it has shown promising efficacy against several viruses in the same Peribunyaviridae family.12
The development of an effective vaccine is also a priority, but studies are scarce. T and B cell epitopes have already been identified from the OROV polyprotein for candidate vaccines.13 In addition, a candidate vaccine has been constructed from vesicular stomatitis virus (VSV) expressing OROV glycoproteins. This vaccine has been evaluated and shown to have a protective effect in vivo, with a reduction in viral load after exposure to the virus.14
Another avenue of research concerns cross-immunity with existing vaccines against other viruses.
Vector control
To date, there are no effective, economically and ecologically feasible vector control measures, particularly for the main vector, Culicoides paranesis. Chemical insecticides such as deltamethrin and N,N-Diethyl-meta-toluamide (DEET) have demonstrated satisfactory insecticidal effects against Culicoides species. However, environmental implications are alarming. Furthermore, given their abundance, complete and permanent eradication of these vectors is impossible. Various natural compounds have been proposed as repellents.
The incompatible and sterile insect techniques are other promising vector control techniques against Aedes spp, and perhaps also against Culicoides.15
Finally, behavioural and sociological research is also needed to develop vector control strategies and reduce the overall risk.
ANRS MIE initiatives
The threat posed by the Oropouche virus should not be underestimated. There are significant gaps in our understanding of its epidemiology, ecology, pathogenesis and the risk of reassortment with other Orthobunyaviruses.
Overcoming this lack of knowledge is therefore essential, as is the development of effective countermeasures to improve risk assessment and devise effective public health strategies for this neglected disease.
A scientific review on Oropouche
ANRS MIE opened a level 1 crisis unit on the Oropouche outbreak in October 2024. A scientific watch is available, updated on a regular basis.
Research projects on Oropouche
ANRS MIE has historically funded the VIROPREG cohort, a cohort on mother-to-child transmission of HIV. The scope of this national prospective cohort has been extended to emerging infectious diseases. VIROPREG plans to study adverse neonatal outcomes and the effects of OROV infection in pregnant women, foetuses and newborns in mainland France and its overseas territories.
Scientific facilitation
In September 2024, the ANRS MIE, in partnership with the Arbo-France network, organised a meeting of experts on the Oropouche virus disease. This meeting provided an opportunity to address:
- Virus circulation in the Americas,
- The risk of introduction into the French overseas territories,
- The Culicoides vector,
- Priority research questions.
References
- Anderson Cr, et al. Oropouche virus: a new human disease agent from Trinidad, West Indies. Am J Trop Med Hyg 1961;10:574-8
- Sah R, et al. Oropouche fever outbreak in Brazil: an emerging concern in Latin America. Lancet Microbe 2024 Jul 3:S2666-5247(24)00136-8
- Gaillet M, et al. Outbreak of Oropouche Virus in French Guiana. Emerg Infect Dis 2021;27(10):2711-2714
- Gómez-Camargo DE, et al. Evidence of Oropouche Orthobunyavirus Infection, Colombia, 2017. Emerg Infect Dis 2021;27(6):1756-1758
- Gutierrez B, et al. Evolutionary Dynamics of Oropouche Virus in South America. J Virol 2020;94(5):e01127-19
- PAHO publishes update on Oropouche fever in the Americas, 2024 Jul. https://www.paho.org/en/news/10-9-2024-paho-publishes-update-oropouche-fever-americas (consulté le 03/10/2024)
- Morrison A, et al. Oropouche Virus Disease Among U.S. Travelers – United States, 2024. MMWR Morb Mortal Wkly Rep 2024;73(35):769-773
- Martins-Filho PR, et al. Oropouche fever: reports of vertical transmission and deaths in Brazil. Lancet Infect Dis 2024:S1473-3099(24)00557-7
- Lee WL, et al. Monitoring human arboviral diseases through wastewater surveillance: Challenges, progress and future opportunities. Water Res 2022;223:118904
- Wesselmann KM, et al. Emergence of Oropouche fever in Latin America: a narrative review. Lancet Infect Dis 2024;24(7):e439-e452
- Recommendations for the Detection and Surveillance of Oropouche in possible cases of vertical infection, congenital malformation, or fetal death, 2024 Jul. https://www.paho.org/en/documents/recommendations-detection-and-surveillance-oropouche-possible-cases-vertical-infection (consulté le 03/10/2024)
- Furuta Y, et al. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol. Sci. 2017;93 :449-463
- Adhikari UK, et al. Immunoinformatics Approach for Epitope-Based Peptide Vaccine Design and Active Site Prediction against Polyprotein of Emerging Oropouche Virus. J Immunol Res 2018;2018:6718083
- Stubbs SH, et al. Vesicular Stomatitis Virus Chimeras Expressing the Oropouche Virus Glycoproteins Elicit Protective Immune Responses in Mice. mBio 2021;12(4):e0046321
- Bansal S, et al. Effectiveness of Wolbachia-mediated sterility coupled with sterile insect technique to suppress adult Aedes aegypti populations in Singapore: a synthetic control study. Lancet Planet Health 2024;8(9):e617-e628
