Mpox
Mpox (formerly known as monkeypox) is a disease that has been circulating for decades in West and Central Africa.
Last updated on 17 January 2025
In brief
Mpox (formerly known as “monkeypox” or “orthopoxvirosis simiana”) is a disease that has been circulating for decades in West and Central Africa. In 2022, for the first time, sustained human-to-human transmission was observed in several countries around the world, including Europe and France.
Origin and spread of the mpox virus
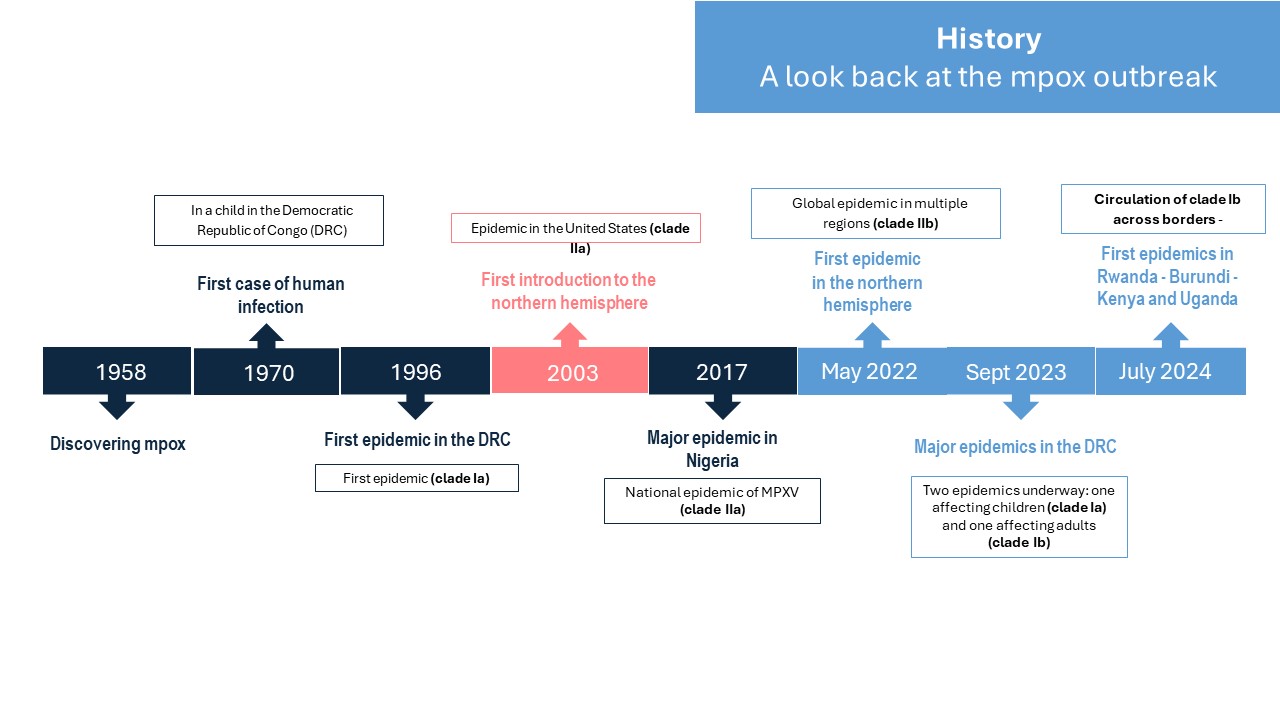
Background: a look back at the mpox outbreak
Mpox disease is caused by the smallpox-like orthopoxvirus MPXV. Identified in 1958 in a laboratory monkey farm in Denmark, the mpox virus was first detected in humans in 1970 in the Democratic Republic of Congo (DRC). The virus has been the cause of increasingly frequent epidemic outbreaks in West and Central Africa. The particularity of the outbreak that began in 2022 is linked to its mode of transmission through sexual contact, which had not been observed in previous epidemics. Since the beginning of 2023, the frequency of epidemics in African regions has been on the rise, particularly in the Democratic Republic of Congo (DRC). This increase in the number of cases has been accompanied by a worrying expansion of the area where the mpox virus is spreading in Central Africa, firstly in new provinces of the DRC, and then in neighbouring countries that had never before documented active circulation of mpox: Burundi, Kenya and Uganda. Surveillance data revealed that these new infections outside Congolese territory were attributable to a new variant of clade I MPXV (sub-lineage Ib). This became a major concern, leading the World Health Organisation to declare the mpox epidemic a public health emergency of international concern (PHEIC) for the second time on 14 August 2024.
History : a look back at the mpox outbreak
Two types of virus: clade I and II
Two clades of the MPXV virus have been described to date: clade I, described since the 1970s in Central Africa, and clade II, present in West Africa.
- Clade I causes more severe forms of the disease, with mortality rates of up to 10% in vulnerable individuals and children (sub-lineage Ia), although the most recent epidemics are associated with lower mortality rates.
- Clade II, whose sub-lineage IIb is at the origin of the global epidemic that began in 2022 (which has primarily affected men who have sex with men (MSM)). Infections by clade II mpox are described with fewer severe forms and fewer deaths.
Transmission of the virus
Human-to-human transmission occurs through direct contact with an infected person, mainly through close skin-to-skin contact with lesions caused by the disease or internal mucous membranes such as the mouth, as well as indirectly through objects contaminated by the patient, such as clothing or bed linen.
Symptoms and treatment of Mpox
The symptoms
The disease manifests itself as a characteristic rash: vesicles and pustules, sometimes large ones, that can cover the whole body, particularly the palms and soles of the feet, face, scalp, anogenital region and mouth. The lesions are very painful. In clade IIb in particular, but also a priori in clade Ib, the lesions are mainly located in the ano-genital area. Other common symptoms include fever, muscle pain and enlarged lymph nodes.
The incubation period is 7 to 17 days. Most patients recover spontaneously after 2 to 4 weeks.
Treatments
Treatment of mpox disease is aimed at curing the rash, reducing pain and preventing complications. Several antivirals have in vitro or in vivo activity against orthopoxviruses, but there is as yet no consolidated data on their efficacy in humans. Three antivirals could be used to treat severe forms of MPXV disease: Tecovirimat, Cidofovir and Brincidofovir. Since 28/01/2022, Tecovirimat has had a European marketing authorisation (MA) in France, granted under exceptional circumstances, and is at the heart of several clinical trials in France and abroad aimed at assessing its efficacy in clade IIb as well as in cases of clade I MPXV infection.
Vaccines
The French National Authority for Health (HAS) updated its vaccination recommendations on 2 September 2024. The two strategies used in 2022 are recommended again:
- one preventive for people who have not been vaccinated or who have been incompletely vaccinated and who are at high risk of exposure to the virus;
- one reactive for people in contact with identified cases within 4 to 14 days.
- Finally, the HAS recommends a booster dose for people vaccinated more than two years ago, born after 1980 and who have not been infected with MPXV since then.
The HAS therefore recommends that people at high risk of exposure to the virus should be eligible for vaccination with the MVA-BN vaccine (Imvanex or Jynneos; third-generation variola vaccines), i.e. :
- Men who have sex with men (MSM) and trans people reporting multiple sexual partners;
- People in prostitution situations ;
- Professionals working in sexual meeting places, whatever their status;
- Partners or persons sharing the same living quarters as the above-mentioned persons.
Vaccines developed against smallpox can be used to protect against MPVX. Real-life efficacy data from studies conducted since the start of the global mpox epidemic in 2022 (clade IIb), in people eligible for vaccination, have shown preventive efficacy of around 80% after two doses.
The French High Council on Public Health (HCSP) has also been asked by French Ministry of Health (Direction Générale de la santé) to issue recommendations on prevention and vaccination against mpox for people travelling to areas where there is active circulation of Clade I mpox. The HCSP considers that the travelers most at risk of contracting mpox in the current context are :
- People who engage in risky sexual practices, regardless of their destination;
- Healthcare professionals and humanitarian workers travelling to an area of active circulation of MPXV clade I (a and/or b), in particular the Democratic Republic of Congo and neighbouring countries in the Great Lakes region;
- People from clade I (a and/or b) MPXV active circulation zones going to visit family and acquaintances;
- Immunocompromised people travelling to areas of active circulation of MPXV clade I (a and/or b).
For these people, the HCSP recommends vaccination with a 3rd generation vaccine
However, questions remain about their effectiveness in real life on clade I, correlates of protection, duration of immunity, efficacy and safety in children and pregnant women. Research projects are therefore needed to better understand and manage the disease.
HAS recommendation HSCP recommendationANRS MIE initiatives
- The ANRS MIE opened a level 1 crisis unit on the mpox epidemic in the DRC in November 2023. A scientific watch is available and is updated on a regular basis.
Since 14 August, the following actions have been carried out:
- Mpox scientific watch published on a weekly frequency
- Scientific coordination implemented to ensure the proper circulation of science based information, promote communication between scientists and French authorities and to discuss research priorities . In this framework several meetings were organized with national and international experts (members of the ANRS MIE International Network) and patient
- Participation of the ANRS MIE in European and international coordination activities
- Responding to the media and disseminating scientific information to the media and the general public
- As part of its commitment to a coordinated response to epidemics, the agency is also pleased to contribute €500,000 to the call for proposals launched by the Global Heath EDCTP 3.
- ANRS MIE is also coordinating the MPX-RESPONSE project. This project is a European Union initiative aimed at strengthening the capacity to respond to mpox epidemics by improving surveillance, early detection, prevention and case management strategies in several European countries.
- The ANRS DOXYVAC trial, sponsored and funded by ANRS MIE, has improved knowledge of vaccination by showing that rapid deployment of smallpox vaccination reduces the risk of mpox.
Mpox Outbreak Response Unit
Status: active – level 1

MPX-RESPONSE project
Enhances Europe’s capacity to provide a rapid response to the mpox international outbreak
ANRS MIE Mpox research priorities
ANRS MIE Research priorities defined in the light of the current situation
- Study of animal reservoirs: identification of the species responsible for spill-over in endemic areas (crossing of the species barrier by the virus, with transmission from animals to humans);
- Development of appropriate molecular and serological diagnostic methods for humans and animals, including the provision of rapid diagnostic tools;
- To gain a better understanding of the dynamics of virus circulation (in animals and humans) and modes of human-to-human transmission: seroprevalence, sexual transmission, droplet transmission, persistence of the virus in biological fluids, study of the duration of contagiousness, modelling study and genomic surveillance in order to anticipate and guide public health policies;
- Natural history and physiopathology of the new clade Ib
- Study in human and social sciences, need for socio-behavioural studies; on appropriate communication strategies to combat stigmatisation and reassure people about the fear of this disease; need for a project on infodaemia;
- Real-life efficacy of the vaccine in clade Ib; durability of the vaccine response; safety and efficacy in children and pregnant women, development of other vaccines
- Treatment efficacy to be assessed by randomised, controlled, multi-country clinical trials
ANRS MIE Mpox news

CROI 2026: Accepted research projects supported by ANRS MIE
CROI, the international Conference on Retroviruses and Opportunistic Infections, will be held from February 22 to 25, 2026, in Denver. Around 20 projects supported by ANRS MIE have been accepted.
23 February 2026
Publications
- Gessain A, et al. Monkeypox. N Engl J Med. 2022.
- Ferré VM, et al. Detection of Monkeypox Virus in Anorectal Swabs From Asymptomatic Men Who Have Sex With Men in a Sexually Transmitted Infection Screening Program in Paris, France. Ann Intern Med. 2022.
- Thy M, et al. Breakthrough infections after post-exposure vaccination against Monkeypox. N Eng l J med. 2022.